Why Does Atomic Number Increase Down a Group
1st IE decreases down the group. Basically as we move down the periodic table the size of the nucleus increases and concomitantly more electrons are present to shield the valence electrons from the charge.

Does Atomic Size Increase Down A Group
This is caused by the increase in the number of protons and electrons across a period.

. Anions are generally larger than cations. The radius of an atom of the element increases from 134 pm to 225 pm Atomic radius increases as you go down the Group 1 elements from top to bottom as an additional energy level electron shell is being added to each successive element. Down a group the number of energy levels n increases so there is a greater distance between the nucleus and the outermost orbital.
In other words the numbe. Why does atomic mass increase as you move down a group in the periodic table. The exra electrons would tends to repel each other to a degree and occupy more space but there is bigger effect-.
Solve any question of Classification Of Elements And Periodicity In Properties with-. Likewise why does atomic radius increase in a group. The number of energy levels increases as you move down a group as the number of electrons increases.
This has to do with the balance of effective nuclear charge put out by the nucleus versus the number of electrons present. Atomic radius is the distance from the atoms nucleus to the outer edge of the electron cloud. One proton has a greater effect than one electron.
If this is the case why would the atomic radii increase if theres more nuclear attractive forces going down a group. So the radius increases as we move down the group. Down a group the number of energy levels n increases so there is a greater distance between the nucleus and the outermost orbital.
Thus electrons are pulled towards the nucleus resulting in a smaller radius. Answer 1 of 27. 類 In general atomic radius decreases across a period and increases down a group.
Atomic radius increases down the group and decreases from left to right in a period. One proton has a greater effect than one electron. In general atomic radius decreases across a period and increases down a group.
In general atomic radius decreases across a period and increases down a group. The atomic number increase down in a group. This is because the number of filled shells increases down the group increasing shielding and the distance between the nucleus and the outermost electrons for very similar effective nuclear charge.
Regarding this why does atomic size increase down a group. Elements of the same period have the same amount of shells and differ only in the number of electrons in. Does atomic radius increase or.
However going across a period also means an increase in both the atomic number and the mass number but in this case the atomic radius decreases. Atomic radius increase in the groups 1-5 and 12-17. Use the data in the table below for Group 17 elements to look for a pattern or trend in.
As we descend a group in the periodic table the atomic number and mass number increase significantly as does the atomic radius. Answer 1 of 4. Down a group atomic radius increases.
類 Click to see full answer. Thus electrons are pulled towards the nucleus resulting in a smaller radius. Therefore the atomic radius increases as the group and energy levels increase.
Down a group atomic. Atomic radius increases from top to bottom within a group. The atomic number increase down in a group.
This results in a larger atomic radius. On moving down the group new shells are being added. We have to keep in mind 3 main points while considering this trend.
Each subsequent energy level is further from the nucleus than the last. Down a group the number of energy levels n increases so there is a greater distance between the nucleus and the outermost orbital. As you go along a period with the atomic number increasing the extra electrons are going in the same shell principle quntum number period number.
Atomic radius increase in the groups 1-5 and 12-17. This is caused by the increase in the number of protons and electrons across a period. The nuclear charge is not enough to reduce the size enough.
Looking at group 1 for example it seems that Zeff remains the same at 1 ie the increase in number of shielding electrons is negated by the increase in protons and consequently atomic radii increases. This means the outermost electron is more loosely held down the group and so less energy is required to remove it. Atomic radius decreases from left to right within a period.
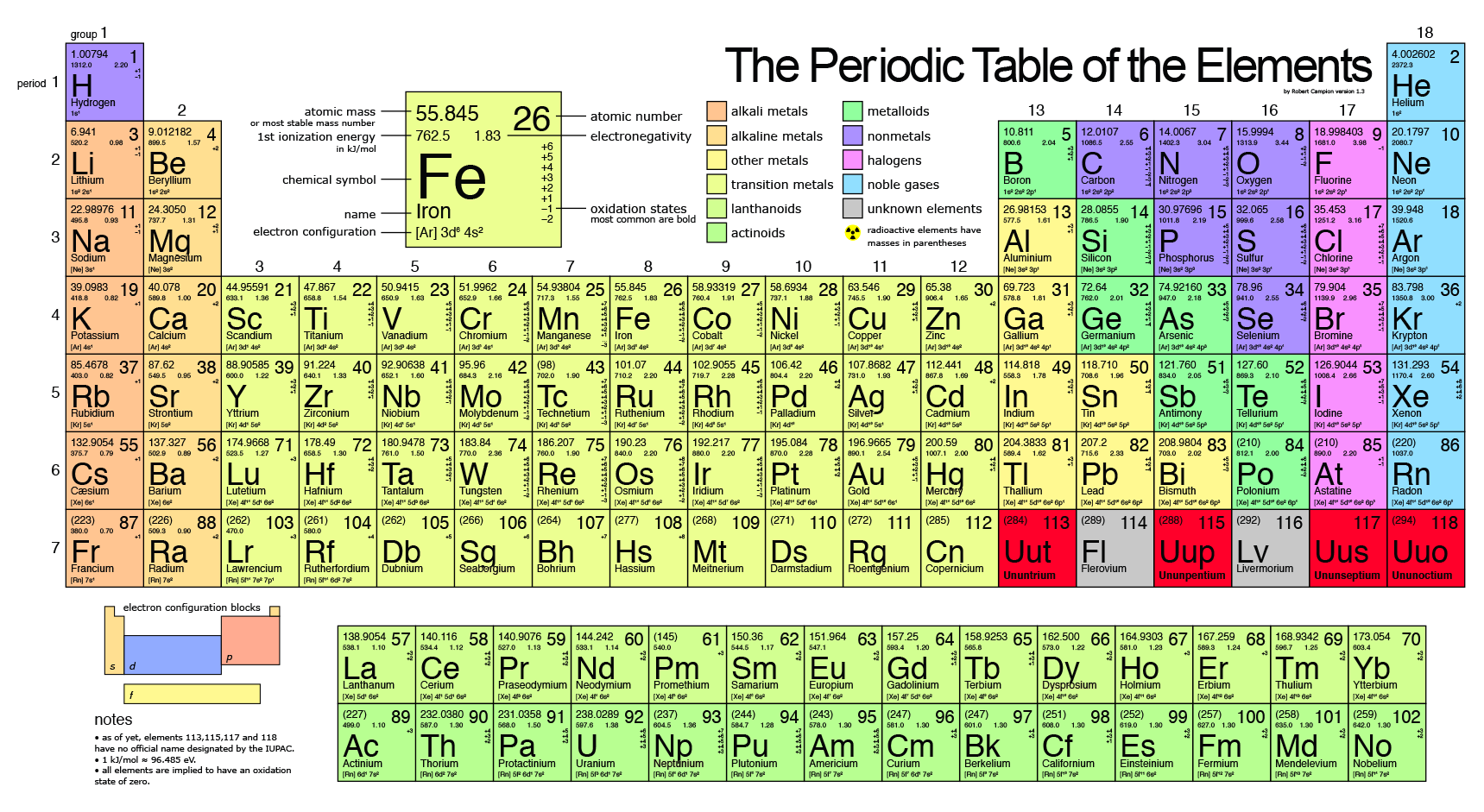
What Happens To The Atomic Mass As You Go Down Each Group Family Socratic
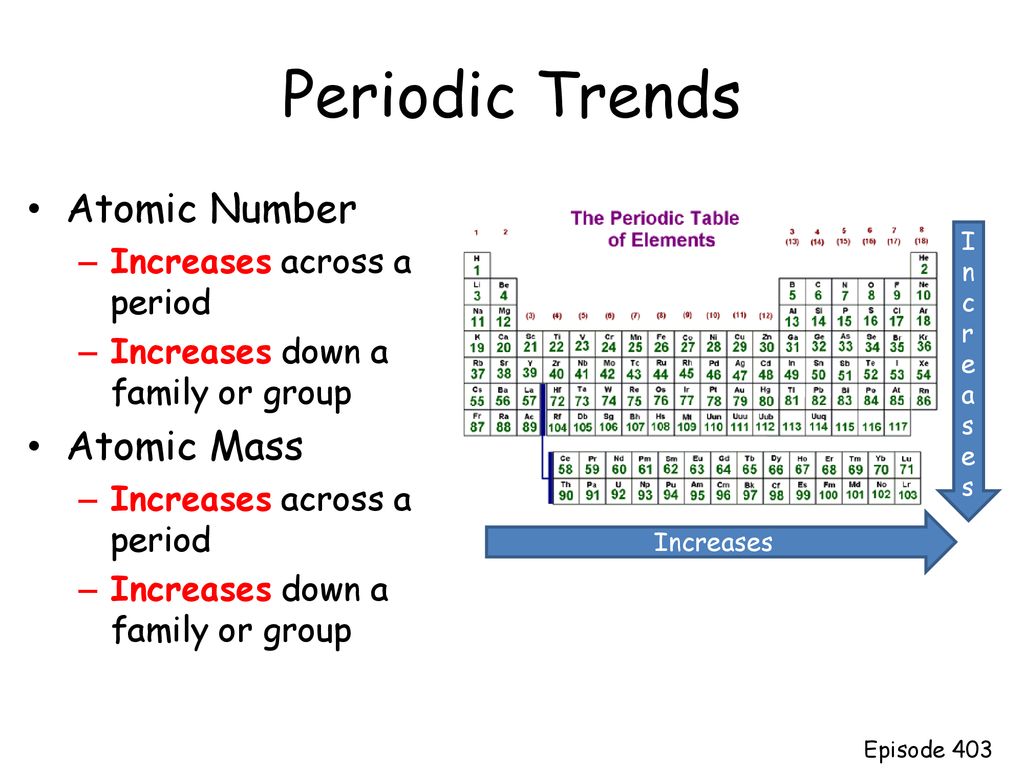
Periodic Trends Atomic Number Atomic Mass Increases Across A Period Ppt Download
Why Does The Atomic Radius Remain Almost Unchanged On The Periodic Table Quora

Periodic Trends Atomic Number Atomic Mass Increases Across A Period Ppt Download
No comments for "Why Does Atomic Number Increase Down a Group"
Post a Comment